Abstract
Background: The acquisition of somatic clonal mutations in the blood of healthy individuals is referred to as clonal hematopoiesis (CH) and is associated with increased age, risk of hematologic malignancy, and non-malignant inflammatory outcomes. The presence of CH in patients with non-Hodgkin lymphoma (NHL) has been associated with adverse outcomes and decreased overall survival (OS) especially in the setting of autologous transplantation. In multiple myeloma (MM), the presence of CH at the time of stem cell collection was associated with decreased OS only in those patients who did not receive thalidomide analog maintenance. However, the clinical significance of CH in patients with newly diagnosed MM undergoing autologous transplant has not been assessed. We sought to determine the frequency and clinical outcome of CH in patients with newly diagnosed MM.
The IFM 2009 study randomized 700 newly diagnosed patients with MM to lenalidomide, bortezomib and dexamethasone (RVd) versus RVd plus high-dose therapy (HDT). This patient cohort provides a unique opportunity to examine the prevalence of CH in newly diagnosed MM patients and assess its influence on clinical outcomes, including early transplantation, in a homogeneously treated population of MM patients.
Methods: We performed targeted error-corrected next generation sequencing of 100 genes recurrently mutated in hematologic malignancies in 377 patients with newly diagnosed MM who were part of the IFM 2009 study (additional patient samples are pending analysis). CH was defined as the presence of any pathogenic mutation with a variant allele fraction (VAF) ≥ 0.005. Sample DNA was collected from peripheral blood of subjects at the time of screening/diagnosis. The median age at sample collection was 59 years. Median follow-up was 7.8 years.
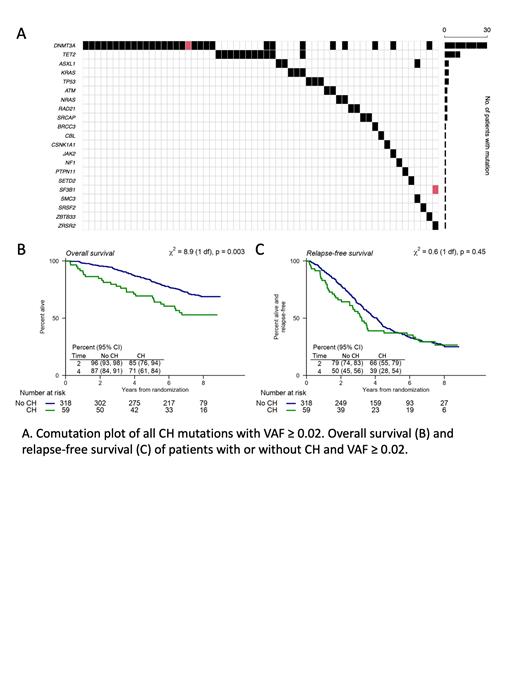
Results: We identified CH in 188 (48%) patients with VAF ≥ 0.005, in 119 (32%) patients with VAF ≥ 0.01 and in 59 (16%) patients with VAF ≥ 0.02. Because prior studies have primarily assessed clinical significance of VAF ≥ 0.02, further analysis was restricted to this threshold. Consistent with prior reports DNMT3A (30 patients), TET2 (11 patients) and ASXL1 (3 patients) were the most commonly mutated genes. The presence of CH was associated with increased median age (61 vs 59 years, p = 0.003), ISS stage III (p = 0.007) and increased LDH (p = 0.006), but not with sex, monoclonal protein isotype, b2-microglobulin level, or assignment to RVd or HDT arms.
As previously reported, randomization to the early transplant arm was associated with increased relapse free survival but not with improved OS. Across the entire cohort, the presence of CH was associated with inferior OS (p = 0.003), but had no association with relapse-free survival or time to relapse. The decrement in OS was seen equally in both the RVd and HDT arms of the study. The presence of mutant clones smaller than a VAF < 0.02 was not associated with adverse clinical outcomes.
Fourteen patients developed a second primary malignancy (SPM), only two of which were hematologic neoplasms. There was no difference in the frequency of SPM development in those patients with or without CH.
Conclusions: In patients with newly diagnosed multiple myeloma, the presence of CH at a VAF ≥ 0.02 is frequently observed and is associated with decreased overall survival regardless of transplantation strategy. The presence of CH did not affect the risk of developing an SPM. Taken together, these data suggest that while CH is associated with decreased survival the presence of CH should not alter eligibility of patients for autologous transplantation.
Sperling: Adaptive: Consultancy. Miller: Foundation Medicine: Consultancy. Moreau: Amgen: Honoraria; Celgene BMS: Honoraria; Abbvie: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; Oncopeptides: Honoraria. Neuberg: Pharmacyclics: Research Funding; Madrigal Pharmaceuticals: Other: Stock ownership. Ebert: Celgene: Research Funding; Skyhawk Therapeutics: Membership on an entity's Board of Directors or advisory committees; GRAIL: Consultancy; Exo Therapeutics: Membership on an entity's Board of Directors or advisory committees; Deerfield: Research Funding. Munshi: Amgen: Consultancy; Abbvie: Consultancy; Takeda: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Oncopep: Consultancy, Current equity holder in publicly-traded company, Other: scientific founder, Patents & Royalties; Pfizer: Consultancy; Adaptive Biotechnology: Consultancy; Karyopharm: Consultancy; Celgene: Consultancy; Legend: Consultancy; Bristol-Myers Squibb: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal